村上定瞭(水浄化フォーラム), Sadaaki Murakami (Water & Solutions Forum)
Basic properties of water
Water and solutes
1.Electrolyte
2.Non-electrolyte
Water and living body constituents
Constituent elements of human body, seawater and crust
1.Electrolyte
(1) Hydration
Electrolyte dissolves in water to form an aqueous solution.
It takes 100 to 1000kJ/mol energy (⊿Gaq) to break the ionic crystal into free ions. On the other hand, the water molecules are polarized, and due to electrostatic interaction between the ions and the water molecules, the water molecules are oriented and stabilized around the ions to become hydrated ions. Hydration also requires energy to break the water structure and orient water molecules.
When the electrolyte dissolves in water, some generate heat and some absorb heat. Free energy is expressed by enthalpy ⊿H and entropy ⊿S, and is expressed by the following equation.
When ⊿H is negative, heat is generated, and when it is positive, heat is absorbed. Regardless of exotherm/endotherm, when ⊿H < T⊿S, ⊿G becomes negative and dissolution reaction occurs. When the free ion hydrates, its hydration energy is almost proportional to z2/r (square of charge/ion radius). That is, the larger this value, the stronger the hydration and stabilization.
Also, when the electrolyte dissolves in water, it dissociates into positive and negative ions, and the electrolyte solution becomes conductive. The greater the degree of hydration, the greater the resistance to movement, and the lower the moving speed of the hydrated ions.
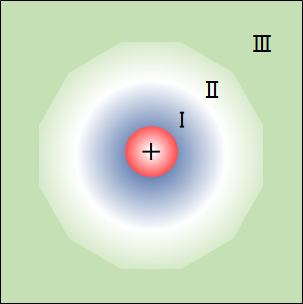
図1 水和イオンと水構造のモデル
Ⅰ:水和した水分子層、Ⅱ:ⅠとⅢの中間層、Ⅲ:氷に似た構造化水相
Fig.1 Model of hydrated ions and water structure.
I: Hydrated water molecular layer, Ⅱ: Intermediate layer between I and III, Ⅲ: Ice-like structured aqueous phase.
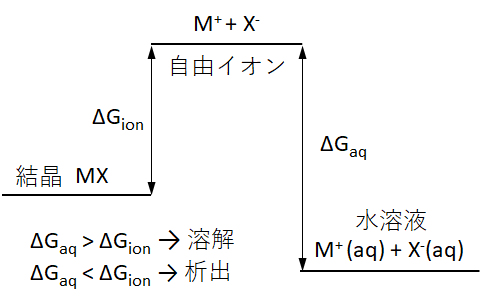
図2 電解質(結晶)が溶解するときの自由エネルギー変化
Fig.2 Free energy change when the electrolyte (crystal) dissolves.
Table 1 ionic hydration energy -ΔG and limit molar conductivity Λ∞.
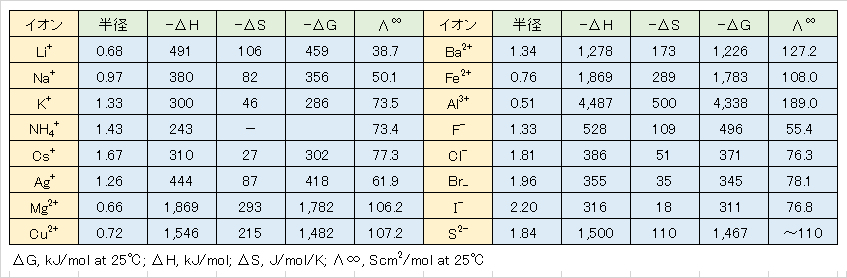
Table 2 Effective ionic radius of inorganic ions in aqueous solution (25℃).
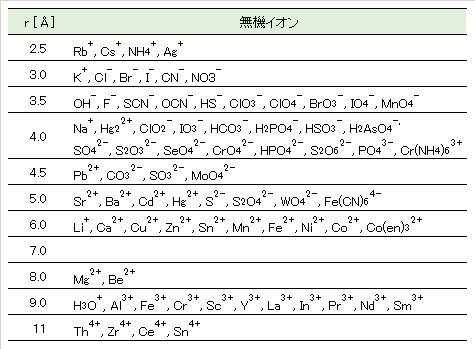
引用文献:日本分析化学会編:分析化学便覧、p.649、丸善出版(2011)
(2)配位水
d軌道やf軌道を有する遷移金属は、配位子が接近すると縮退しているd軌道やf軌道が分裂し、低スピン型では低準位の軌道に電子が配置されて安定化する(配位子場安定化エネルギー)。遷移金属イオンに対して水分子が配位子として働き、[M(OH2)n]z+(aq)として安定化する。
例えば、塩化コバルト(Ⅱ)には、無水塩(青色)をはじめ、数種の水和塩が存在し、安定な六水和塩は単斜晶(桃色)である。塩化コバルト(Ⅱ)無水塩の水溶液と塩化コバルト(Ⅱ)六水和塩の結晶の吸収曲線は一致している。このことから、コバルト(Ⅱ)イオンは水溶液中では水和塩結晶と同じように6個の水分子で正八面体形に囲まれていることを示している。この電解質の水溶液は桃色であるが、塩酸を加えていくと桃色から青色へ変化することはよく知られている。
コバルト(Ⅱ)の外殻電子は3d7であり、6個の水分子が配位することで、d軌道はdε(dxy、dyz、dzx)とdγ(dx2-y2、dz2)に分裂し、 外殻電子がdε5dγ2(正八面体形、高スピン型)に配置されてコバルト(Ⅱ)錯体が安定化する。[CoCl4]2-(aq)の電子配置はdγ4dε3(正四面体形、高スピン型)となる。[F.A. Cotton and G. Wilkinson: Advanced Inorganic Chemistry, p.565, p.881, John Wiley & Sons(1972)]
(2) Coordination water
Transition metals with d or f orbitals are stabilized by degenerate d or f orbitals when the ligand approaches, and electrons are placed in low level orbits in the low spin type to stabilize. (Ligand field stabilization energy). The water molecule acts as a ligand for the transition metal ion, and the generated complex ion is stabilized as [M(OH2)n]z+(aq).
For example, cobalt (II) chloride has several kinds of hydrated salts including anhydrous salt (blue), and stable hexahydrated salt is monoclinic (pink). The absorption curves of the aqueous solution of anhydrous cobalt (II) chloride and the crystals of cobalt (II) chloride hexahydrate are in agreement. From this, it is shown that the cobalt (II) ion is surrounded by an octahedron shape with six water molecules in the same manner as the hydrated salt crystal in the aqueous solution. The aqueous solution of this electrolyte is pink, but it is well known that pink changes from pink to blue when hydrochloric acid is added.
The outer shell electrons of cobalt (II) are 3d7, and the coordination of six water molecules results in the d orbital dε (dxy, dyz, dzx) and dγ (dx2-y2, dz2), and the outer electrons are dε5dγ2 (regular octahedral, high spin type). The electronic configuration of [CoCl4]2-(aq) is dγ4dε3 (regular tetrahedron type, high spin type). [F.A. Cotton and G. Wilkinson: Advanced Inorganic Chemistry, p.565, p.881, John Wiley & Sons(1972)]
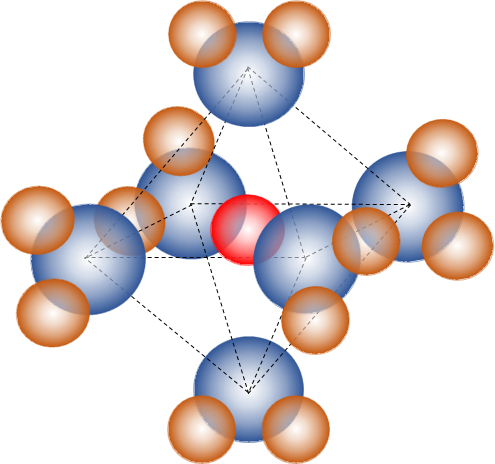
図3 [Co(OH2)6]2+錯イオンの構造(赤球:Co2+、青球:O、茶球:H)
Fig.3 [Co(OH2)6]2+ complex ion structure (red sphere: Co2+, blue ball: O, brown ball: H)
非電解質
(1)小さい分子
非電解質にはファンデルワールス力が働き、メタンやエタンなどの低分子の物質は水構造の隙間に入り込んで水に溶け込む。しかし、電解質に比べ、溶ける量は極めて少ない。
(2)大きな分子や粒子
親水基と疎水基を有する大きな分子は、疎水部分を内側に畳み込み親水部分を水と接触させて、水に溶け込む。パンパク質・DNAなどの生体高分子が水に溶け込む例が代表的なものである。また、親水基と親油基を有する界面剤は、親油基を油粒相へ親水基を水相へ向けてミセルを形成し、油粒を水相へ分散させる。
Non-electrolyte
(1) Small molecule
The van der Waals force acts on the non-electrolyte, and low-molecular substances such as methane and ethane enter the gaps of the water structure and dissolve in water. However, as compared with the electrolyte, the amount of dissolving is extremely small.
(2) Large molecule and particle
A large molecule having a hydrophilic group and a hydrophobic group is dissolved in water by folding the hydrophobic part inward and bringing the hydrophilic part into contact with water.A typical example is that biopolymers such as bread crumbs and DNA dissolve in water. In addition, an interfacial agent having a hydrophilic group and a lipophilic group forms micelle and disperses oil particles in the aqueous phase
by directing the lipophilic group to the oil grain phase and the hydrophilic group to the aqueous phase.
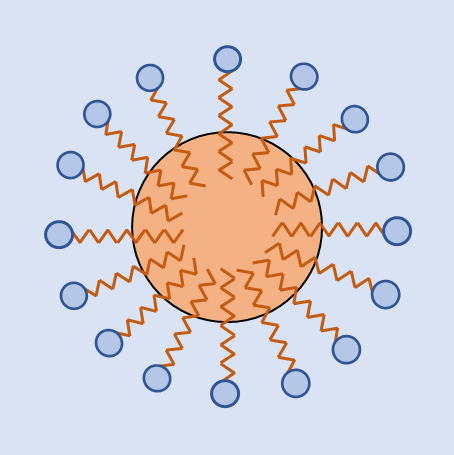
図4 油粒のミセル Fig.4 Oil micelle
掲載:2017年07月03日
更新:2020年07月04日(英語版追加)
Posted: July 03, 2017
Update: July 04, 2020 (English version added)
