Outline of water purification technology – Removal of suspended substances
村上定瞭(水浄化フォーラム), Sadaaki Murakami (Water & Solutions Forum)
懸濁性物質の除去
水処理では有機・無機を問わず汚濁物質を水に不溶性の固形物の形に変えて水と分離するのが原則である。例えば、重金属イオンの除去ではpH調整による水酸化物沈殿又は陰イオン添加による不溶性塩を析出させ、固液分離する。
水中の汚濁物質は密度差を利用して沈降または浮上させて除去する。また、ろ材を充填した層内に水を通してこの層内に懸濁物質を捕捉させたり、あるいは微細な孔を通して懸濁粒子の通過を阻止して取り除くといったろ過がある。ろ過の中には、コロイド粒子から分子・イオンまでも除去する膜分離がある。さらに、水を蒸発して溶解物質や懸濁物質を乾固して取り出す方法もある。
Removal of suspended substances
In water treatment, it is a rule to convert pollutants into organic and inorganic solids that are insoluble in water and to separate them from water. For example, in the removal of heavy metal ions, hydroxide precipitation by pH adjustment or insoluble salt by anion addition is precipitated, and solid-liquid separation is performed.
Contaminants in water are removed by sedimentation or floating using the density difference. There is also filtration in which water is passed through a layer filled with a filter medium to trap suspended substances in this layer, or suspended particles are prevented from passing through fine pores to be removed. Filtration includes membrane separation that removes molecules and ions from colloidal particles. Furthermore, there is also a method of evaporating water to dry out a dissolved substance or a suspended substance and taking out.
1.沈降分離
水よりも密度が大きな物質を、水との密度差を利用して沈降させる方法である。水を一定の場所に長時間滞留させるか、非常にゆっくりと流して浮遊物質を沈殿させる。沈降分離は普通沈殿と後で述べる凝集分離とに分けられる。普通沈殿は凝集沈殿操作を施さずにそのまま沈降分離させるもので、自然沈降とも呼ばれる。
普通沈殿における固形物の分離効率は、固形物の沈降速度分布と装置の表面負荷によって決まる。上昇流式沈殿池では、沈降速度が水の上昇速度より大きい粒子はすべて沈降分離され、水の上昇速度より小さい粒子はすべて流出する。懸濁物質の沈降速度分布を求めて、沈殿池の設計を行う。
なお、沈降分離については、沈殿池の基礎で詳しく解説しているので、参考とされたい。
1. Sedimentation Separation
A method in which a substance having a density higher than that of water is allowed to settle by utilizing the density difference with water. Allow the water to stay in place for a long time or run very slowly to settle the suspended solids. Sedimentation separation can be divided into ordinary precipitation and coagulation separation described later. Ordinary precipitation is a method in which sedimentation is performed without performing a coagulation sedimentation operation, and is also called spontaneous sedimentation.
The separation efficiency of solids in ordinary precipitation depends on the sedimentation velocity distribution of solids and the surface load of the device. In an upflow sedimentation basin, all particles with a sedimentation rate higher than that of water are sedimented and separated, and all particles with a sedimentation rate less than that of water flow out. Design the sedimentation basin by obtaining the sedimentation velocity distribution of suspended solids.
For sedimentation separation, it is explained in detail in ‘Basics of Settling Tank/Basin‘, so please refer to it.
2.凝集分離
水中の浮遊物質の粒径が小さく、前述の自然沈殿では処理に長時間を要し、処理効率が悪い場合に行われる。一般に水中に浮遊している微粒子の表面は帯電している。これに反対電荷を持つ薬品を添加して、目的粒子の電荷を中和すると、粒子間の引力が表面荷電による反発力を上回るようになり微粒子同士が凝集して大きな塊(フロック)へと成長していく。このような目的に用いられる薬品を凝集剤と呼ぶ。
水処理には安価で無害である鉄又はアルミニウムの無機塩類が用いられる。高分子凝集剤は少量の添加量で凝集効果があり、しかも大きなフロックができる特徴がある。高分子凝集剤には、陽イオン性、陰イオン性及び非イオン性に分類され、さらにその分子量や分岐によって多くの種類があり、懸濁物質の種類により選択する。
なお、凝集分離については、分散・懸濁微粒子で詳しく解説しているので、参考とされたい。
2. Coagulation separation
It is carried out when the particle size of suspended solids in water is small and the above-mentioned natural precipitation requires a long processing time and the processing efficiency is poor. Generally, the surface of fine particles floating in water is charged. When a chemical with the opposite charge is added to this to neutralize the charge of the target particles, the attractive force between the particles exceeds the repulsive force due to surface charge, and the particles agglomerate to grow into a large lump (flock). I will do it. A chemical used for such a purpose is called a flocculant.
Inorganic salts of iron or aluminum, which are inexpensive and harmless, are used for water treatment. The polymer flocculant has a feature that it has a flocculating effect even when it is added in a small amount and large flocs are formed. Polymeric flocculants are classified into cationic, anionic and nonionic, and there are many types according to their molecular weight and branching, and they are selected according to the type of suspended substance.
Regarding aggregation and separation of dispersion/suspension particles, it is explained in detail in another page, so please refer to it.
3.浮上分離
水中の懸濁物質の密度が水より小さければ、水面に浮くことになるので、浮上させて分離することが可能である。対象物質としては、油類がその代表的なものである。
また、密度が水よりも大きい懸濁物質であっても、空気の泡を懸濁物質に付着させれば、速やかに浮上する。水中に微細な空気を発生されるには、空気をいったん加圧して水に溶解してから大気圧に解放して微細な気泡を発生させる加圧浮上法の他に、水の電気分解による水素や酸素の微細気泡を利用する方法もある。浮上分離においては、コロイド状の懸濁粒子は分離できないので、あらかじめ凝集処理をしておく必要がある。
3. Levitation separation
If the density of suspended solids in water is smaller than that of water, it will float on the surface of the water and can be separated by floating. Oil is a typical example of the target substance.
Also, even if the suspended substance has a density higher than that of water, if air bubbles are attached to the suspended substance, it quickly rises. In order to generate fine air in water, in addition to the pressure levitation method that pressurizes air once to dissolve it in water and then releases it to atmospheric pressure to generate fine bubbles, hydrogen by electrolysis of water There is also a method of using fine bubbles of oxygen. In the floating separation, colloidal suspended particles cannot be separated, so it is necessary to perform a coagulation treatment in advance.
4.清澄ろ過
重力式分離(沈降、浮上)で除去できなかった微量の懸濁物質を、さらに清澄な水を得るのが清澄ろ過である。ろ材としては一般に砂が用いられるので、砂ろ過とも呼ばれる。砂ろ過では懸濁粒子はろ材間の空隙に捕捉される。ろ過を続けているうちに、ろ過抵抗が上昇しろ過水の濁度も上昇してくる。ろ過抵抗又はろ過水濁度のいずれかが設定値に達したら、ろ過を中断してろ材を充填した層(ろ層)を洗浄する。この洗浄をろ層の再生という。原水中の懸濁物質の濃度が高いと短時間でろ層が閉塞するので、一般には重力式分離の後に砂ろ過を行う。
4. Clarification filtration
Clarification filtration is used to obtain more clear water from a trace amount of suspended substances that could not be removed by gravity separation (settlement, floatation). Since sand is generally used as the filter medium, it is also called sand filtration. In sand filtration, suspended particles are captured in the voids between the filter media. As the filtration continues, the filtration resistance increases and the turbidity of the filtered water also increases. When either the filtration resistance or the filtered water turbidity reaches the set value, the filtration is interrupted and the layer filled with the filter medium (filter layer) is washed. This washing is called regeneration of the filter layer. When the concentration of suspended solids in the raw water is high, the filter layer is blocked in a short time, so sand filtration is generally performed after gravity separation.
5.膜分離
図1に示すように極めて微細な穴を持つ膜を通して水をろ過し、細菌のようなコロイド次元の大きさの懸濁固形物から溶解性物質やイオン物質に至る不純物を除去する技術を膜分離法という。使用する穴の大きさによって、精密ろ過(MF)、限外ろ過(UF)、ナノろ過(NF)、逆浸透(RO)などがある。これらはろ過の駆動力として圧力差を用いている。その他、直流電圧を駆動力とし、イオン交換膜(アニオン膜、カチオン膜)を用いる電気透析法がある。
5. Membrane separation
As shown in Figure 1, water is filtered through a membrane with extremely fine holes, and impurities from suspended solids of colloidal dimensions such as bacteria to soluble substances and ionic substances The technology for removing the impurities is called a membrane separation method. Depending on the size of the holes used, there are microfiltration (MF), ultrafiltration (UF), nanofiltration (NF), reverse osmosis (RO), etc. These use the pressure difference as the driving force for filtration. In addition, there is an electrodialysis method that uses a DC voltage as a driving force and uses an ion exchange membrane (anion membrane, cation membrane).
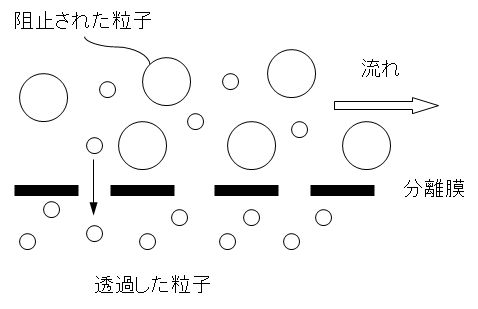
図1 分離膜による微細粒子の分離機構
Figure 1 Separation mechanism of fine particles by separation membrane.
ナノろ過は、限外ろ過と逆浸透の中間に位置するものであり、逆浸透に比べて小さい駆動力で低分子の物質を除去することができる。
水は透過するが、溶質はほとんど透過しない性質を持った膜を逆浸透(半透)膜という。この膜を介して水と溶液を置くと、水だけが溶液側に移動し、ある高さで平衡に達する。このときの水位差を浸透圧といい、溶液の濃度が高いほど高くなる。このとき溶液側に浸透圧以上の圧力をかけると、水溶液側の水だけが半透膜を透過して水側に移動する。このようにして、無機塩類や低分子有機物の水溶液から水だけを取り出すことが可能となる。これが逆浸透膜の原理である。
膜を要素技術として装置化したものを膜モジュールという。現在実用化されている膜モジュールには平膜型、スパイラル型、チューブラー型、中空糸型などがある。平膜型は膜を装着した透水性の多孔板の両面にスペーサーを介して多数重ね合わせたもの、スパイラル型は多孔性支持材を内蔵した封筒状の膜をのり巻き状に巻き込んだもの、チューブラー型は多孔性の耐圧支持管の外側又は内側に膜を装着したもの、中空糸は外径1300μm内径700μmくらいのマカロニ状の細長い中空の膜を多数束ねたものである。
なお、分離膜と微細粒子除去および膜分離活性汚泥法の実験については、別途記載してあるので参考にされたい。
Nanofiltration is located between ultrafiltration and reverse osmosis, and can remove low-molecular substances with a smaller driving force than reverse osmosis.
Water is permeable, but solute is almost impermeable. It is called reverse osmosis (semi-permeable) membrane. When water and solution are placed through this membrane, only water moves to the solution side and reaches equilibrium at a certain height. The difference in water level at this time is called osmotic pressure, and the higher the concentration of the solution, the higher. At this time, if a pressure higher than the osmotic pressure is applied to the solution side, only water on the aqueous solution side permeates the semipermeable membrane and moves to the water side. In this way, it is possible to extract only water from the aqueous solution of the inorganic salt or the low molecular weight organic substance. This is the principle of the reverse osmosis membrane.
Membrane module is a device made by using the membrane as an element technology. The membrane modules currently in practical use include flat membrane type, spiral type, tubular type, and hollow fiber type. In the flat membrane type, a large number of water-permeable porous plates with membranes are superposed on both sides with a spacer interposed therebetween. The spiral type is a film in which an envelope-shaped film containing a porous support material is wound into a roll. In the tubular type, a membrane is attached to the outside or the inside of a porous pressure-resistant support tube. The hollow fiber is a bundle of many macaroni-shaped elongated hollow membranes having an outer diameter of 1300 μm and an inner diameter of 700 μm.
Note that separation membrane and fine particle removal and Experiment of membrane separation activated sludge method is described separately, so please refer to it.
6.蒸発乾固
濃厚溶液の場合には、水分を蒸発させて懸濁性及び溶解性の両成分を乾固させる方法が用いられる。水分の蒸発には加熱法と冷凍法がある。水分の蒸発には、多大なエネルギーを必要とするが、汚濁物質が濃厚(重量濃度が数~数十%)場合や有効な処理方法のない汚濁物質の処理に有効である。
6. Evaporation to dryness
In the case of concentrated solution, a method of evaporating water to dry both the suspending and soluble components is used. There are heating method and freezing method for evaporation of water. Evaporation of water requires a large amount of energy, but it is effective when the pollutant is concentrated (weight concentration is several to several tens %) or when there is no effective treatment method.
